Mg OSTEOCRETE
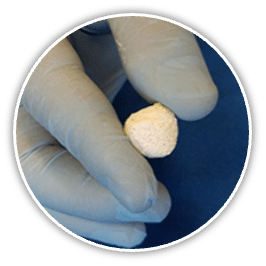
Mg OSTEOCRETE is a fast setting Magnesium-based bone substitute that remodels into bone over time through creeping substitution.
Injectable, Moldable
Magnesium-Based
Bone Void Filler
Magnesium phosphate biomaterial with a unique combination of high strength, osteoconductive, stimulative, and bonding characteristics.
Mg OSTEOCRETE has a unique resorption profile that provides stability, increasing cell proliferation and advancing mineralization, resulting in enhanced bone regeneration in a broad range of orthopedic applications.
Mg OSTEOCRETE bone substitute is made from a pre-measured blend of magnesium, phosphates, and a pre-measured proprietary solution. When mixed and molded/injected according to the instructions for use, Mg OSTEOCRETE will harden in situ at the defect site.
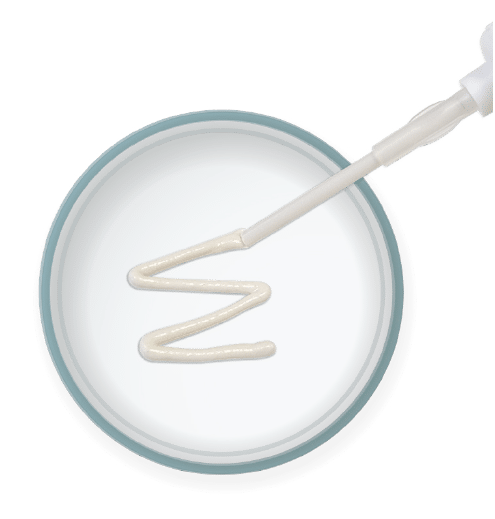
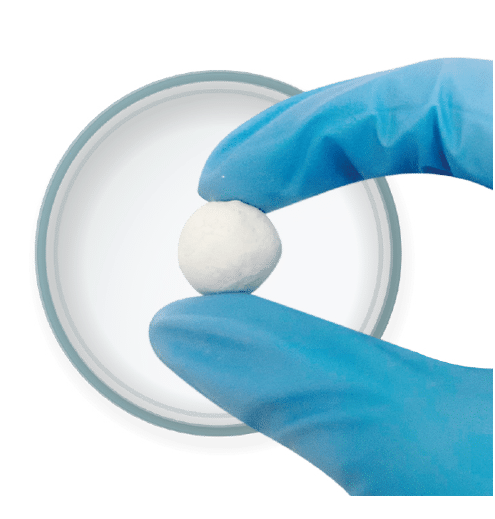
Injectable & Moldable
Optimized consistency for use in multiple orthopedic applications
Radiopaque
Product easily identifiable in situ
Fully Synthetic Material
Enhanced quality control and product availability with reduced patient morbidity
Osteoconductive/ High Compressive Strength
Surface topography to support bone formation, enhancing structural stability and biocompatibility
Temperature Setting Control
Allows for optimal workability and curing time
Excellent Binding Characteristics
Optimal product stability and fixation at the operative site
Thixotropic Properties
Easily manageable curing time allowing for intraoperative flexibility and reduction of waste
Orthopedic Surgery Enhanced Bone Regeneration
Greater than 80% bone remodeling in 26 weeks*
What is Mg OSTEOCRETE™?
Mg OSTEOCRETE™ is a combination of magnesium oxide, monopotassium phosphate, and tricalcium phosphate powder mixed with a modified saline and forms an osteoconductive and biodegradable cement. Unique to Mg OSTEOCRETE™ is its’ ability to adhere to bone. Mg OSTEOCRETE’s™ patented, proprietary formula includes critical components needed to help maximize bone health and development.
The Patented, proprietary processes provide a unique combination of osteoconductive and high compression strength characteristics, delivering an ideal non-weight-bearing orthopedic implant.
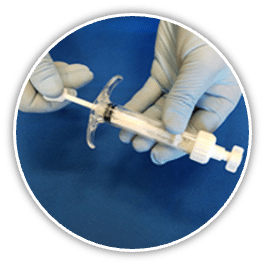
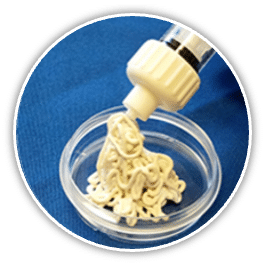

- Excellent Handling and Ease of Use
- Radiopaque to facilitate injection
- Biocompatibility and Safety
- Available in 5cc, 10cc, and 15cc kits
- Can be molded to fit uncontained bone voids
- Will resorb into both cancellous and cortical bone
- Will provide 3d structure
Why Magnesium?
- Critical for bone health and development.
- The central dogma of molecular biology is based on transcribing RNA from DNA and translating protein from RNA, none of which happens without the presence of magnesium.
Mg OSTEOCRETE™ Highlights
Magnesium technology has three (3) times the compression strength (36 MPAs) of cancellous bone with adhesive qualities of PMMA for short-term fixation.
Injectable or moldable and drillable after 6 minutes of implantation with a work time for moldability of 1 hour in the OR.
100% resorbed by the body in 3-10 months, depending on the patient’s health, implant mass, and site location.
Only product on the market that can be reconstituted to have zero financial waste.
Every known scientific study comparing a magnesium phosphate to a calcium phosphate bone void filler has shown improved/quicker bone remodeling for the magnesium phosphate product. This includes studies utilizing OsteoCrete® and independent basic science studies evaluating the properties of magnesium phosphate.
Testimonials
“This study demonstrates that Mg OsteoCrete® has short-term fixation similar to that of PMMA bone cement and may, therefore, provide sufficient mechanical support of a metaphyseal implant in patients with poor bone quality where augmentation of implant fixation is desired.”
Joseph P. Iannoti MD, PHD, Cleveland Clinic
Orthopedics. 2015 Jul 1;38(7):e597-603. doi: 10.3928/01477447-20150701-58.
“Bone Graft Substitute Provide Metaphyseal Fixation for a Stemless Humeral Implant.”
Kim MS, Kovacevic D, Milks RA, Jun BJ, Rodriguez E, DeLozier KR, Derwin KA, Iannotti JP.
“The results of this study indicate that a magnesium-based bone adhesive can improve tendon-to-bone healing. It may eventually be possible to use this bone adhesive clinically to augment tendon-to-bone healing, potentially leading to increased attachment strength and a diminished risk of graft failure or slippage.”
Scott A. Rodeo MD, Hospital for Special Surgery
Am J Sports Med. 2008 Jul;36(7):1290-7. doi: 1177/0363546508314396. Epub 2008 Mar 4.
“Augmentation of tendon-to-bone healing with a magnesium-based bone adhesive.”
Gulotta LV1, Kovacevic D, Ying L, Ehteshami JR, Montgomery S, Rodeo SA.
Backed Research
- Passive resorption (dissolution) MgO and K Struvite are not highly water-soluble components, so magnesium ions are slowly eluted.
- Active resorption (cell-mediated) surrounding cells break down the bone graft and replace it with bone.
- Mg OSTEOCRETE™stimulates this process via magnesium ion release
- Mg OSTEOCRETE™ had 83% greater resorption than Norian at 12 weeks (p<0.001)
- 84% of Mg OSTEOCRETE™ was resorbed within 26 weeks and was replaced with woven or lamellar mineralized bone of normal morphology
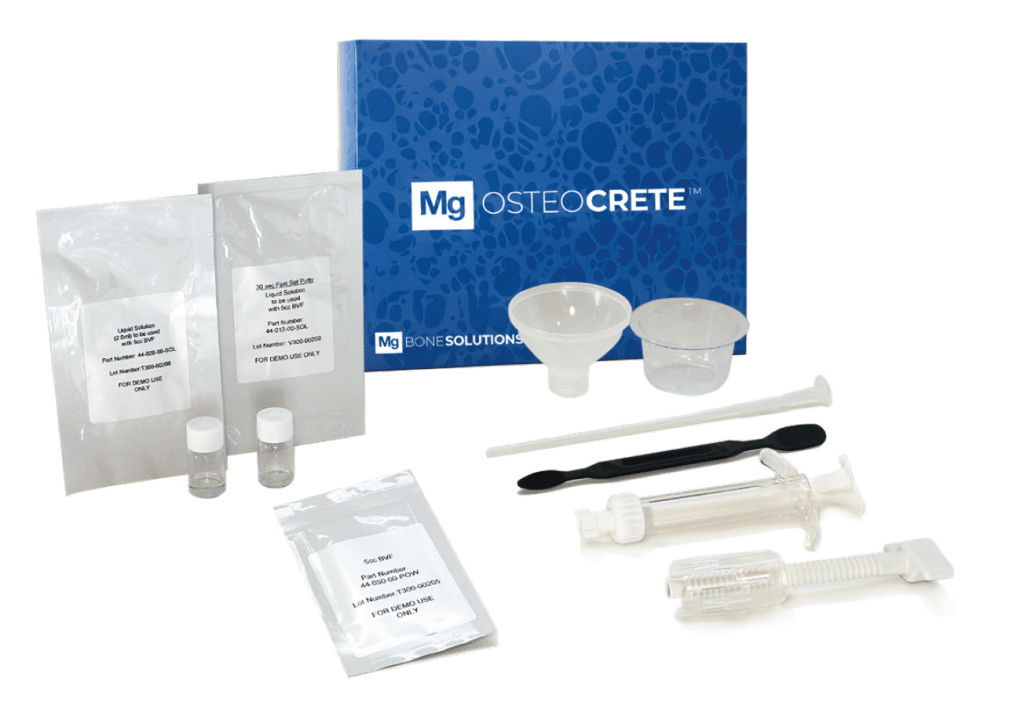
Mg OSTEOCRETE Full Kit
Kit Contents: 5cc Mg Powder, (1) Vial Liquid Solution for 30-second putty, (1) Vial Liquid Solution for injectable, Mixing Syringe, Funnel, Basin, Spatula & 4.2mm Cannula/Pusher, Mechanical Advantage
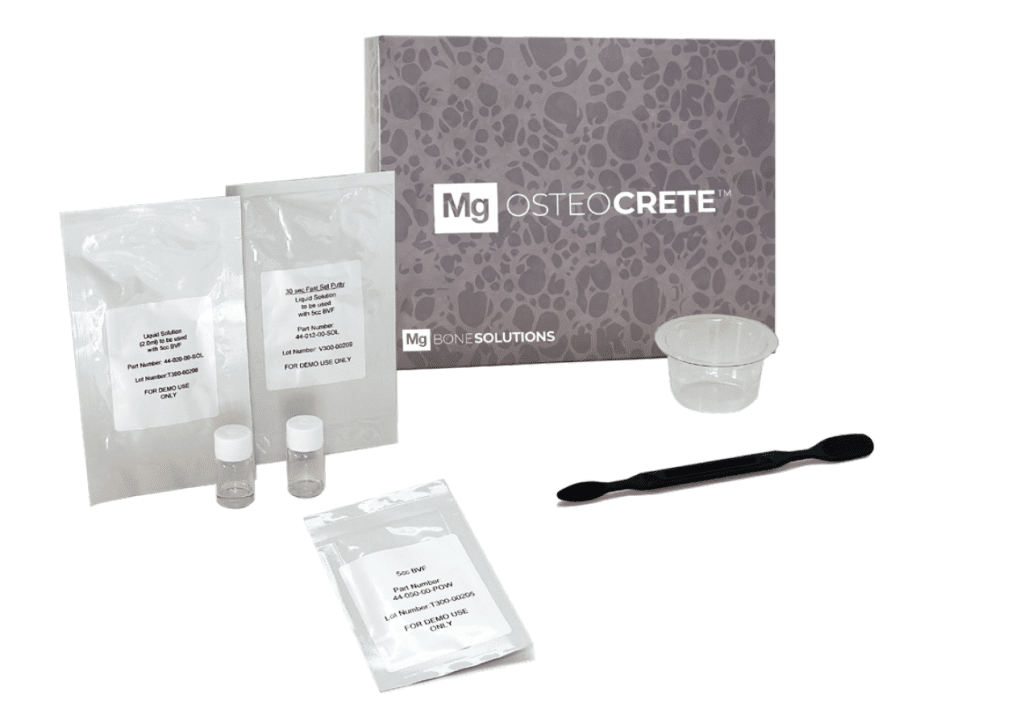
Mg OSTEOCRETE Basic Kit
Kit Contents: 5cc Mg Powder, (1) Vial Liquid Solution for 30-second putty, (1) Vial Liquid Solution for injectable, Basin, Spatula

MIXING & DELIVERY SYSTEM
Kit Contents: A delivery system that provides a complete mixing of Mg OSTEOCRETE and excellent visibility for precise graft placement.

Bead Mat & Scraper
Kit Contents: (1) Bead Mat, (1) Scraper

Aspiration Needle
Kit Contents: (1) Bone Marrow Aspiration Needle
Delivery Cannula
Kit Contents: (1) Aspiration Needle
Mg OSTEOCRETE is a fast setting Magnesium-based bone substitute that remodels into bone over time through creeping substitution.
Product Specifications
| Part Number | Description |
|---|---|
| 44-050-00-BSI | Full Kit – 5cc |
| 44-100-00-BSI | Full Kit – 10cc |
| 44-150-00-BSI | Full Kit – 15cc |
| 44-050-00-STR | Basic Kit – 5cc |
| 44-100-00-STR | Basic Kit – 10cc |
| 44-150-00-STR | Basic Kit – 15cc |
| 44-000-00-AUX | Mixing & Delivery System |
| RM-44-050 | Bead Mat & Scraper |
| 74174-15M | Bone Marrow Aspiration Needle 8Ga X 150mm |
| 74066-01M | Bone Marrow Aspiration Needle 11Ga X 110mm |
| 74358-01M | OsteoINJECT 11Ga CLOSED TIP Drillable Side Port Delivery Cannula |
| 74357-01M | OsteoINJECT 11Ga OPEN TIP Drillable End Port Delivery Cannula |
